The Find-AF story
by Rolf Wachter
The study design of Find-AF 2 has been significantly influenced by two precursor studies, Find-AF I and Find-AFRANDOMISED. Find-AF I was a purely observational study, Find-AFRANDOMISED was the randomised pilot and dose-finding study of Find-AF 2.
The Find-AF story began in July 2008, when Dr Klaus Gröschel1 and Prof Andreas Kastrup2 met for the first time. Back then, I proposed a joint study on the investigation of natriuretic peptides for the detection of atrial fibrillation in stroke patients (Find-AF I).
_________________________________
- At that time assistant physician in neurology at the University Medical Center of Göttingen, today managing senior physician in neurology at the University Medical Center of Mainz
- At that time senior physician in neurology, today head physician in neurology at the Bremen-Mitte Clinic.
Find-AF I 2009-2010
On 21 March 2009, we were able to include the first patient in the study and by 20 February 2010, 281 patients had been included. On 2 June 2010, we were able to present the results for the first time at the neurological colloquium in Göttingen and the manuscript was accepted in "Stroke" a few weeks later. The central statement of the paper is shown in Figure 1.
Figure 1: Detection rate of atrial fibrillation in Find-AF I. The bars indicate the atrial fibrillation rate on the respective monitoring day, the line shows the cumulative detection rate. Original slide from the presentation on 02.06.2010. / Graphic: Wachter/Wasser
Based on the data of the Find-AF I study, we are applying with the DFG for the Find-AF 2 study for the first time in 2010. The submitted outline was judged controversially, as the very different reviewer comments show. In the end, however, we were invited to submit a full proposal.
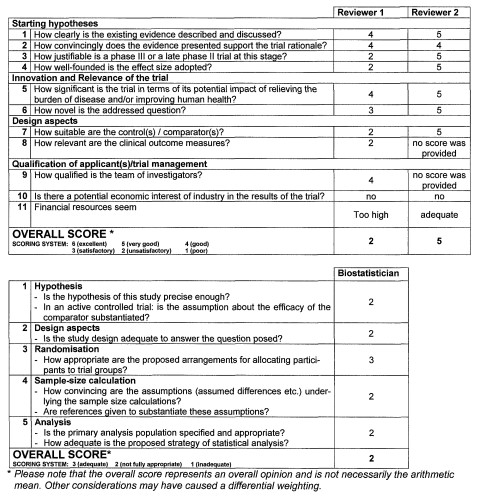
Figure 2: Assessment of the Find-AF II abstract in the DFG letter dated March 16, 2011. The clinical reviewers rate on a scale of 1-6, with 6 being the best rating. Biometricians rate on a scale of 1-3, with 3 being the best. / Graphic: Wachter/Wasser
With such widely divergent assessments, the DFG Clinical Trials Panel took a close look at the study and asked us to submit a full proposal. The panel's reasoning is shown in Figure 3.

Figure 3: Panel ‘comments and statement’ from DFG letter dated March 16, 2011. / Illustration: Wachter/Wasser
We thus prepared a full proposal, which we submitted on time on May 26, 2011. The plan was for 2550 patients to receive either a 7-day long-term ECG after randomization followed by repeated 3-day long-term ECG examinations at 3, 6, and 12 months or an implantable event recorder. The study design of that time is shown in Figure 4.
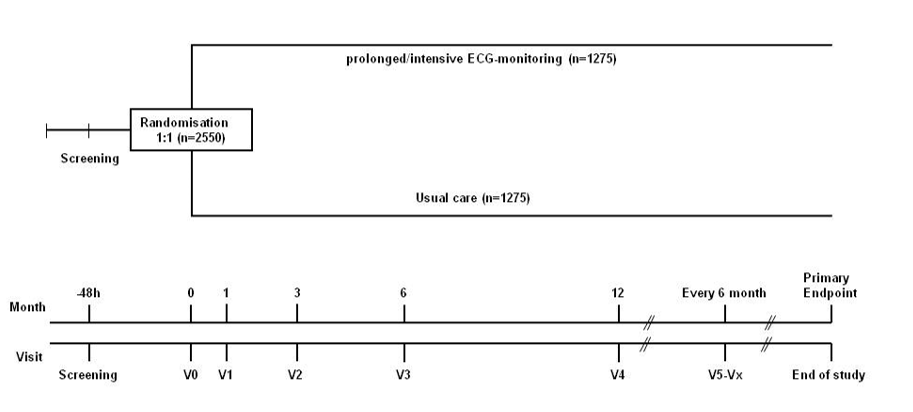
Figure 4: Study design of the Find-AF II study (1st full application 05/2011) / Figure: Wachter/Wasser
A follow-up of 3 years was planned on average, the total budget requested was € 3,427,333.
On October 28, 2011, the DFG wrote informing us that the review had unfortunately been negative. Three clinical and one biometric reviewer had assessed the study with again very different results. The DFG panel's reasoning for rejection at that time was:
Panel statement:
Comments: "This study aims at comparing prolonged ECG monitoring versus usual care (2-4 yrs follow-up) for patients who have had a recent ischemic stroke to prevent a 2nd event. The primary outcome is time to recurrent stroke. It is a large study of N=2550 large trial recruiting from 20 centres. The proposed costs is very large. The follow-up is 2-4 years (36 months mean).
Implicitly the study in evaluating not just the diagnostic value of the prolonged monitoring, but also the subsequent treatment.
This is an important area for research, but the applicant’s design will not provide clear answers to the question. The main issues are as follows: The selection of patients needs to be clearly defined. For example, patients with stroke in whom imaging shows multiple infarcts in different cortical territories on MRI is much more likely to have AF as the underlying cause of their stroke. The patient with extensive small vessel disease and a lacunar stroke is very unlikely to have AF as the underlying cause. Hence, all patients must have standard investigations and either the applicants concentrate on including patients most likely to have a cardiac source, or if they wish to include all patients, then they must stratify the randomisation by clinical stroke sub-type and analyse the date by these subgroups.
The applicants need to address the fact that trials of warfarin versus aspirin in patients not known to have AF or other cardiac sources of embolism (e.g. WARSS and ESPRIT) have failed to show any benefit of warfarin above aspirin. If there were significant numbers of patients with covert AF that would benefit from warfarin in the way the applicants propose, then one would have expected the trials to show superiority of warfarin over aspirin.
Hence, the applicants have first to show whether or not patients who are found to have a covert and short episode of AF on prolonged monitoring soon after stroke, are at increased risk of subsequent cardio-embolic stroke. The likelihood is that the occurrence of AF shortly after stroke may, in many patients, be it a response to damage to the brain (infarction of the insular cortex is associated with ECG changes, for example) or a response to metabolic instability (e.g. post-stroke pneumonia) and therefore may be unlikely to recur and cause subsequent stroke recurrence.
The applicant’s proposal to allow patients in the monitoring arm to have either Holter monitoring or an implanted rhythm monitor is not an acceptable compromise. They should study one or the other only, since the yield of the two methods will be very different, and the side effects and costs are also very different.
The treatment of AF is rapidly changing and thrombin inhibitors are likely to be used more and more over the next few years. Any trials in which most patients get warfarin are therefore likely to be seen as out-dated even before they are published. This is even more reason to first find out if the AF they detect with longer monitoring is important risk factor or not.
Taken together, the study cannot be recommended for funding."
Panel decision: reject
A short comment from today's point of view (10 years later):
Perhaps the time was not ripe enough for Find-AF 2 at that time. We were later able to answer the question as to whether or not we should limit ourselves to cryptogenic strokes (the answer is no) with Find-AF randomised. The question of whether insular infarcts or pneumonias can cause passagenic AF without an increased risk of stroke remains unclear but is probably only a marginal issue.
We subsequently made several more attempts to obtain funding for Find-AF 2 from the DFG (and also once from the German Center for Cardiovascular Research), but were ultimately always unsuccessful.
However, in summer 2012 we were able to convince the company Boehringer Ingelheim to fund the Find-AFRANDOMISED study. At the end of 2012, we were able to sign the contracts and as of Jan. 1, 2013, we started planning for the study, which we then named Find-AFRANDOMISED. Dr. Mark Weber-Krüger, who had participated in the Find-AF I study as a doctoral student, could be convinced to medically coordinate the study.
Find-AFRANDOMISED (2012-2017)
Patients were recruited for Find-AFRANDOMISED from May 2013 to August 2014. We had opted for an oligocentric study-concept with four major study centres (University Medical Centre Göttingen, University Medical Centre Mainz, Hospital Sanderbusch and HSK Clinics Wiesbaden).
The study design of the Find-AFRANDOMISED study is shown in Figure 5.
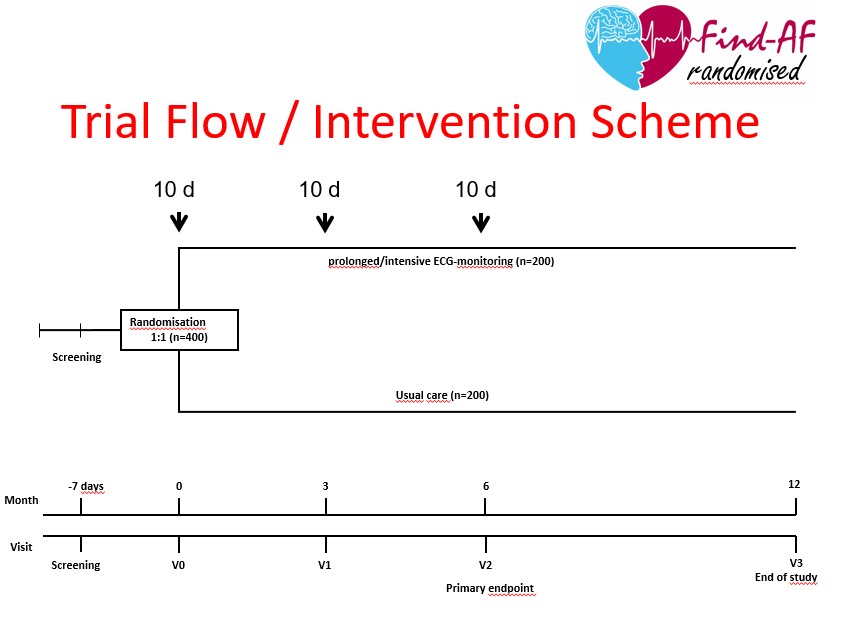
Figure 5: Study design of the Find-AF_RANDOMISED study / Graphic: Wachter/Wasser
One year later, the follow-up was completed and we submitted the results in autumn 2015 as a "late breaking clinical trial" for the International Stroke Conference 2016 in Los Angeles. Fortunately, the study was accepted and I was able to present the results as a late breaking clinical trial at the opening session of the International Stroke Conference 2016 in Los Angeles (Figure 6).

Figure 6: Presentation of the results of the Find-AFRANDOMISED study at the International Stroke Conference 2016 in Los Angeles / Photo: Klaus Gröschel
The results were also published in "Lancet Neurology" almost a year later. Figure 7 shows the primary endpoint atrial fibrillation and the secondary endpoint stroke in the Kaplan-Meier representation.
Figure 7: More atrial fibrillation and fewer recurrent strokes in the Find-AF_RANDOMISED group of patients who received three 10-day ECGs (red) compared to the control group of patients who received standard diagnostic and treatment (blue). Modified after Wachter et al., Lancet Neurology 2017 / Graphic: Wachter/Wasser
What was also important to us was to inform the study participants about the results. Therefore, shortly after the publication of the study results, we invited all study participants from Göttingen (originally 155) as well as interested lay people to the University Medical Center Göttingen to present the results to you. You can see in the picture (Figure 8) the largest lecture hall (Lecture Hall 81), which I have never seen as full as it was on that day.

Figure 8: Presentation of the results of the Find-AF_RANDOMISED trial for trial participants and interested parties. Göttingen, 26 July 2017 / Image: Rolf Wachter
With the results of the Find-AFRANDOMISED study, we again applied for funding for the Find-AF 2 study from the German Research Foundation and, after several obstacles, we finally received the funding decision for Find-AF 2 on 20 September 2019. We then worked at high pressure to draw up the study protocol and finally, on 6 July 2020, the first two patients could be registered in the Find-AF 2 study in Leipzig.


